Epigenetics in Cilia and Cardiac Development
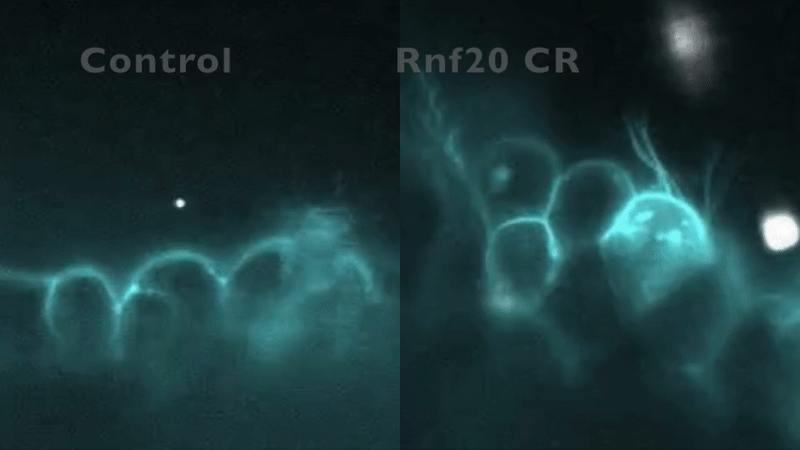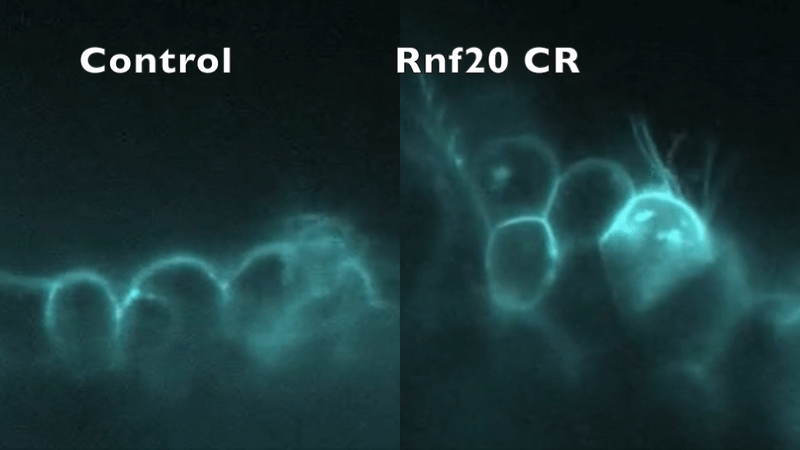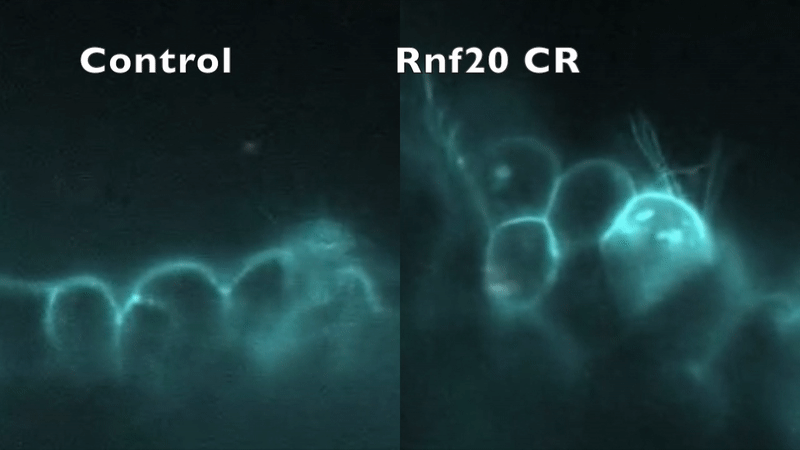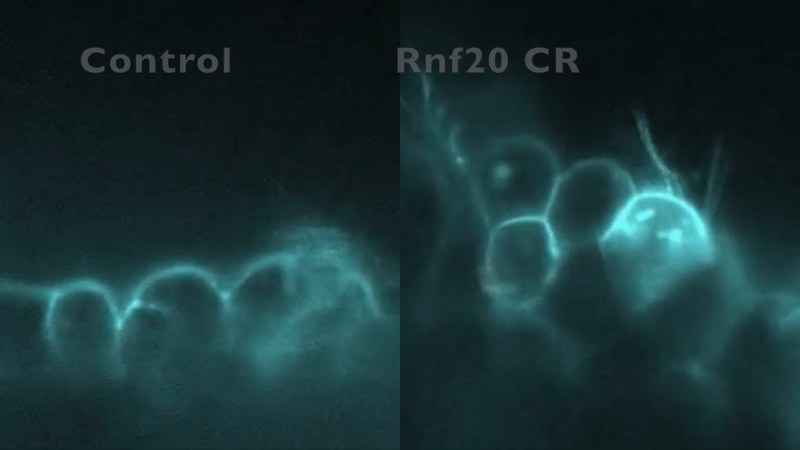
CRISPR depletion of RNF20 hinders cilia motility and fluid flow, as indicated by tracking fluorescent beads. Robson et al. (2019).
Whole exome sequencing on 2,426 parent-offspring trios with severe CHD in the offspring allowed us to identify a highly significant excess of de-novo dominant mutations in chromatin remodeling genes, including 11 patients with mutations affecting monoubiquitylation of Histone H2BK120. But, as in many cases of these discoveries, the mechanisms linking mutation to disease are unknown.
In our preliminary work, knockdown of five CHD-associated chromatin remodeling genes in Xenopus produced an entirely unexpected phenotype: in addition to abnormal cardiac development, cilia structure and/or motility were abnormal.
This mirrored the phenotype of a patient with a mutation in RNF20, who had heterotaxy and primary ciliary dyskinesia (PCD) with absent inner dynein arms. RNF20 is an E3 ubiquitin ligase that ubiquitylates H2BK120. We were able to demonstrate that RNF20 regulates cardiac left-right development via transcriptional control of cilia motility at the left-right organizer. However, RNF20 is broadly expressed, including in the developing heart outside of the LRO. We propose that RNF20 functions in cardiac development first through control of the transcriptional program directing cilia biogenesis and LR patterning, and then through direct function in the heart.
Current experiments are testing the mechanism of RNF20-mediated H2BK120 ubiquitylation in mouse embryos and cultured cardiomyocytes using ChIPseq and RNAseq analyses.
Selected Publications:
Picture Credit: Acetylated tubulin (cilia marker, red) in the mouse node. Picture by Svetlana Makova.